Calcium is more of a nuisance than a “contaminant”. These are not a health hazard. The crystals are easily distinguished from broken glass or plastic when viewed under low magnification. Chemical identification is straightforward & simple with the right tools. The standard field/spot test uses dilute acid (it fizzes from released carbon dioxide).
The recommended daily intake is around 1000 mg for adult males. The amount in the bottle is much much less than that. A liter/quart of hard water may contain 100 mg calcium. A Tums tablet (used both as an antacid and calcium supplement) contains 500 -750 mg calcium carbonate.
Nature exploits calcium’s borderline solubility, using it for seashells, eggshells, pearls. Stalactites and stalagmites underground. Hard water deposits that build up inside faucets, on glass shower doors, showerheads, requires periodic replacing of the washer in faucet valves that develop drips. (Similarly, teeth and bones are made of calcium phosphate in a collagen protein matrix. But animal horns, claws, fingernails are largely keratin protein, not calcium)
Chemical: The Group 2 alkaline earth metals are divalent cations (magnesium, calcium, strontium, barium) that are present in the Earth’s crust. Calcium is the 5th most abundant element in the Earth’s crust. Magnesium is 8th. Strontium is 15th. Their solubility is generally much lower than the Group 1 alkali metals (sodium, potassium). Seawater contains 25X more sodium than calcium. On the other hand, ground water usually contains more calcium than sodium (excluding brine deposits).
3 types of crystal structure:
calcite = rhombohedral (slanted cube)
aragonite = needle shaped
vaterite = amorphous
Calcium can be 100 ppm and above in hard water (milk is ~ 1000 ppm or 0.1% Ca)
The recommended daily amount of calcium is 1000 milligrams/day for an adult male. Of all the minerals, only potassium and sodium are needed in a larger amount than calcium.
=======================================================================
(Below) Crystalline sediment can be seen from underneath, but not from the side or above


Crystals captured on 47 mm filter disc

Below: Close-up photo. Two crystal forms of CaCO3 are visible; the needle shaped aragonite, and the ‘slanted cube’ calcite.
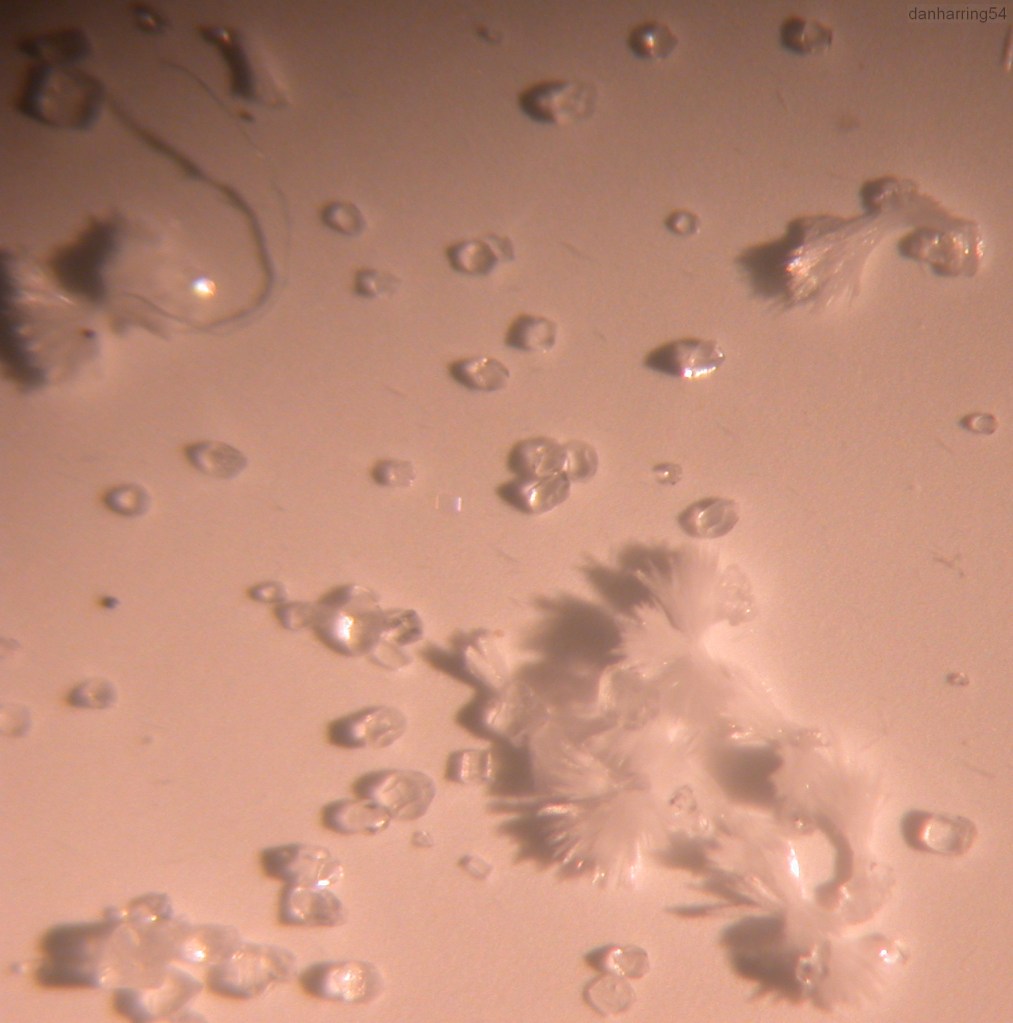
Rhombohedral calcite Xtals (seen by holding an early digital camera to eyepiece of a low-power stereo zoom microscope)
